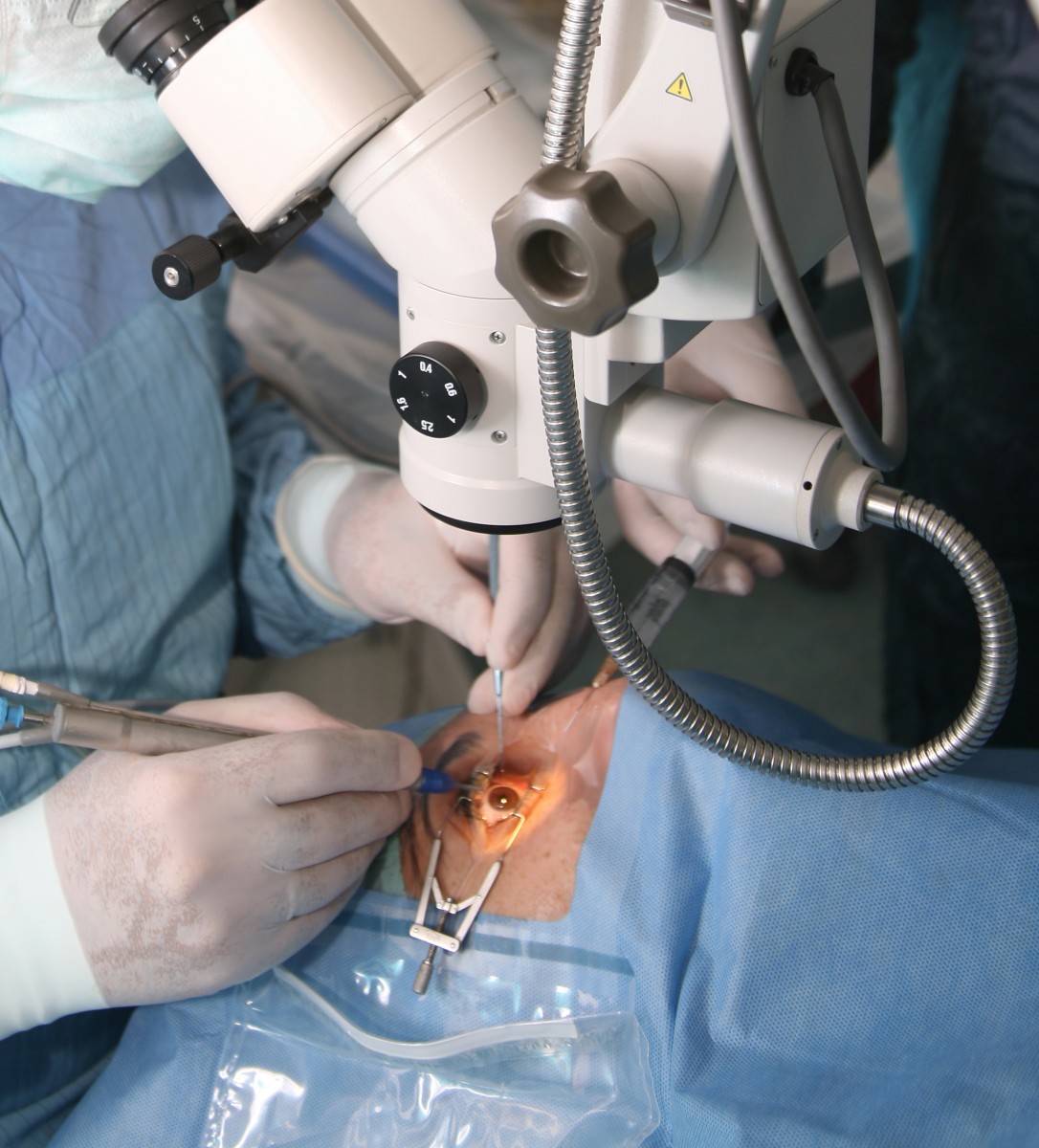
 Icon Bioscience Inc., a biopharmaceutical company focused on developing and commercializing ophthalmic pharmaceuticals based on its proprietary Verisome drug delivery technology, recently announced positive data regarding their Phase 3 trial that examined the efficacy of IBI-10090 in the treatment of inflammation following cataract surgery. Results from the prospective, double-blind randomised Phase 3 multicentre study revealed that IBI-10090 is safe and effective.
Icon Bioscience Inc., a biopharmaceutical company focused on developing and commercializing ophthalmic pharmaceuticals based on its proprietary Verisome drug delivery technology, recently announced positive data regarding their Phase 3 trial that examined the efficacy of IBI-10090 in the treatment of inflammation following cataract surgery. Results from the prospective, double-blind randomised Phase 3 multicentre study revealed that IBI-10090 is safe and effective.
The results will be presented during the Annual Symposium & Congress of the American Society of Ophthalmic Administrators (ASOA) and the American Society of Cataract and Refractive Surgery (ASCRS) on the 19th of April.
Icon Bioscience’s presentation tiled “Safety and Efficacy of a Novel Intracameral Dexamethasone Drug Delivery Suspension for Treating Inflammation Associated With Cataract Surgery,” is co-authored by Wendy Murahashi, MD from Icon Bioscience, Eric D. Donnenfeld, MD from Long Island Ophthalmic Consultants and Edward J. Holland, MD from the Cincinnati Eye Institute.
The study is published in ASCRS.
IBI-10090 uses the Verisome technology to deliver into the eye’s anterior chamber a precise and a sustained release of dexamethasone, an anti-inflammatory agent that is administered after cataract surgery. Each year more than 3 million cataract procedures are performed in the United States, and since the care associated with inflammation causes a similar burden, “IBI-10090 addresses a clear medical need in a large ophthalmic pharmaceutical space,” said David S. Tierney, MD, Icon’s President & CEO, in a press release.
Dr. Tierney noted that, “Icon is pursuing a 505(b)(2) regulatory approval route for IBI-10090, reflecting a strategy of reducing product development risks by applying the Company’s unique Verisome technology to significantly expand the therapeutic value of an already approved drug entity.”
The active agent of IBI-10090, dexamethasone, is a commonly prescribed drug both in generic and branded medications. The drug, which belongs to the class of ophthalmic drugs, can be found in product offerings from Alcon eye-care as well as Allergan in the division of Novartis AG and the Bausch & Lomb operations of Valeant Pharmaceuticals.
“As IBI-10090 moves out of the pipeline toward marketing approval, Icon will realize significant growth milestones,” said Tierney. “The potential regulatory approval of IBI-10090 in the foreseeable future will strongly validate and enhance the value of our Verisome drug delivery platform while positioning the Company for growth as a commercial operation. This is truly an exciting time for Icon.”